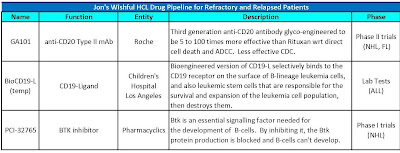
I was going to call this post "A Little Bit of Everything", but fortunately my plans have changed!
I did it!, or should I say "Dr. Kreitman and The NIH Trial to Treat First Time HCL Patients did it!" All of my tests are now negative: peripheral blood flow (looks for hairies in your veins), bone marrow and most importantly bone marrow aspirate (the liquid in your bones where cells develop) are all negative! I couldn't have done it without the NIH trial. If you recall, it's taken me a little over two years to get here.
The initial treatment with Cladribine only led to a partial remission, and I still had 30% bone marrow infiltration and positive blood flows at 6-months post-chemo (November 2009). At that point, I had my first Rituxan treatment (8 rounds over 8 weeks). I achieved a complete remission (CR) 6 months later, in May 2010, but still had hairy cells in my bone marrow aspirate (a value not included in determining CR). That 1st CR was short lived. The cells in my aspirate continued to proliferate and upon restaging in December 2010, my bone marrow infiltration was back up to 5% and the peripheral blood flow showed .04% hairies. Although my complete blood counts (CBCs) were at remission levels, the marrow infiltration indicated it was no longer a CR and positive flow results for hairy cell indicated minimal residual disease (MRD), so I was retreated with Rituxan for the second and final time in accordance with the trial's protocol. This latest treatment wiped out the hairies in all areas to undetectable levels. I'm not completely out of the woods. Time will tell if this is a durable remission, but one thing's for sure: The combination of Cladribine and Rituxan did what Cladribine alone could not without waiting for relapse or subjecting me to multiple rounds of chemo.
Although I've wiped out the hairies, I have one more minor hurdle to jump. As I discovered and wrote last year, there is a documented phenomenon that occurred in 20% of lymphoma patients, who received combined chemo and Rituxan therapy, known as Rituxan-related late onset neutropenia (LON). The nadir (low point in counts) typically occurs 14 weeks after the last dose of Rituxan when the counts dip to about 660 (average). The effect lasts for about 4 weeks. I noticed a similar effect happened to me after my first Rituxan treatment and blogged about it then. You'll also recall that I've been interested in seeing if it would happen again. Well, it happened again! My 6-month CBC (16 weeks after my last Rituxan dose or 24 weeks after I started this cycle) indicates that my neutrophils are at 750. Although it appears to be slightly later by 2 weeks, I don't get CBCs often enough to say whether the low-point occured at 14 weeks or not.
Still, it is statistically in keeping with the findings of the lymphoma study. I believe I've discovered and am the first documented case of this occuring in an HCL patient. Time will tell as my recovery and other patients are studied. I expect to be at a remission level in 3 weeks, when I have my next CBC. So technically, I'm not in a CR until the neuts jump back up, but I don't really care. Everything else is in great shape. Based on my first experience and knowledge of RTX LON, I consider it a nuance that will quickly recover; however, I won't be surprised if the duration of this neutropenia is slightly longer than the first time.
So far it's been a great summer. My youngest daughter, who was born a year after I was first treated, started walking on her first birthday and can now say the letters in her name. My 3-year old is doing great and having lots of fun with her garden and playing in the sand and taking swim lessons. We recently headed up to Lancaster, PA to visit Dutch Wonderland and the Thomas the Train event at Strasburg railroad in our new Swagger Wagon (a Honda Odyssey).
The sun and I have also come to terms. I'm going to wear sunscreen and s/he's going to lay off me for awhile. Likewise, I've set up a portable 60 Watt solar panel array to charge all my mobile devices and run my low power electronics to pay her/him hommage. Yes, I'm a gadget geek, but if the power ever goes out, I'll still have my iTunes! I also just bought a kayak and will be taking lots of late afternoon trips on the Potomac this summer.
Yippeekayay !!!
I did it!, or should I say "Dr. Kreitman and The NIH Trial to Treat First Time HCL Patients did it!" All of my tests are now negative: peripheral blood flow (looks for hairies in your veins), bone marrow and most importantly bone marrow aspirate (the liquid in your bones where cells develop) are all negative! I couldn't have done it without the NIH trial. If you recall, it's taken me a little over two years to get here.
The initial treatment with Cladribine only led to a partial remission, and I still had 30% bone marrow infiltration and positive blood flows at 6-months post-chemo (November 2009). At that point, I had my first Rituxan treatment (8 rounds over 8 weeks). I achieved a complete remission (CR) 6 months later, in May 2010, but still had hairy cells in my bone marrow aspirate (a value not included in determining CR). That 1st CR was short lived. The cells in my aspirate continued to proliferate and upon restaging in December 2010, my bone marrow infiltration was back up to 5% and the peripheral blood flow showed .04% hairies. Although my complete blood counts (CBCs) were at remission levels, the marrow infiltration indicated it was no longer a CR and positive flow results for hairy cell indicated minimal residual disease (MRD), so I was retreated with Rituxan for the second and final time in accordance with the trial's protocol. This latest treatment wiped out the hairies in all areas to undetectable levels. I'm not completely out of the woods. Time will tell if this is a durable remission, but one thing's for sure: The combination of Cladribine and Rituxan did what Cladribine alone could not without waiting for relapse or subjecting me to multiple rounds of chemo.
Although I've wiped out the hairies, I have one more minor hurdle to jump. As I discovered and wrote last year, there is a documented phenomenon that occurred in 20% of lymphoma patients, who received combined chemo and Rituxan therapy, known as Rituxan-related late onset neutropenia (LON). The nadir (low point in counts) typically occurs 14 weeks after the last dose of Rituxan when the counts dip to about 660 (average). The effect lasts for about 4 weeks. I noticed a similar effect happened to me after my first Rituxan treatment and blogged about it then. You'll also recall that I've been interested in seeing if it would happen again. Well, it happened again! My 6-month CBC (16 weeks after my last Rituxan dose or 24 weeks after I started this cycle) indicates that my neutrophils are at 750. Although it appears to be slightly later by 2 weeks, I don't get CBCs often enough to say whether the low-point occured at 14 weeks or not.
Still, it is statistically in keeping with the findings of the lymphoma study. I believe I've discovered and am the first documented case of this occuring in an HCL patient. Time will tell as my recovery and other patients are studied. I expect to be at a remission level in 3 weeks, when I have my next CBC. So technically, I'm not in a CR until the neuts jump back up, but I don't really care. Everything else is in great shape. Based on my first experience and knowledge of RTX LON, I consider it a nuance that will quickly recover; however, I won't be surprised if the duration of this neutropenia is slightly longer than the first time.
So far it's been a great summer. My youngest daughter, who was born a year after I was first treated, started walking on her first birthday and can now say the letters in her name. My 3-year old is doing great and having lots of fun with her garden and playing in the sand and taking swim lessons. We recently headed up to Lancaster, PA to visit Dutch Wonderland and the Thomas the Train event at Strasburg railroad in our new Swagger Wagon (a Honda Odyssey).
The sun and I have also come to terms. I'm going to wear sunscreen and s/he's going to lay off me for awhile. Likewise, I've set up a portable 60 Watt solar panel array to charge all my mobile devices and run my low power electronics to pay her/him hommage. Yes, I'm a gadget geek, but if the power ever goes out, I'll still have my iTunes! I also just bought a kayak and will be taking lots of late afternoon trips on the Potomac this summer.
Yippeekayay !!!