The Rituxan treatments have been an overwhelming success!
While I haven't taken the time to write a worthwhile update on my current status, I'd like to share with you the flow results from blood taken prior to the Week 5 treament. The flow was negative -- no hairy cells could be detected using the standard FACS, which is sensitive enough to detect 1 hairy in 10,000 mononuclear cells.
The Cladribine/Rituxan one-two punch worked and was the perfect combination of what I needed. I'm very relieved and very grateful to have found Dr. K's trial. I've now completed 7 of the 8 weekly Rituxan treatments. The last treatment went very well and didn't require Tylenol or Benadryl pre-meds (to eliminate undue strain on my liver).
A week prior, the treating nurse gave me my Tylenol pre-meds half an hour before drawing my blood for the chem20 tests, which include my liver enzyme levels. Not surprisingly, both the AST and ALT enzyme levels were about as high as I'd seen in awhile. This week, the AST was back to normal and the ALT was 50% higher than normal, which isn't too bad for me. I had a consult with a hepatologist, who wasn't surprised that in general my enzymes were elevated. It is not uncommon in half of all cancer patients, given the stress of chemotherapy and all the other agents the body is exposed to. I don't know if that generalization applies to Cladribine chemotherapy specifically, but it helped me feel better about my roller coaster enzyme levels.
Based on prior imaging tests, it appears that my liver is somewhat fatty, and the hepatologist seems confident that a better diet and more exercise will likely return the liver to normal. I'll continue to persist at maintaining dietary and physical discipline in hopes that the fatty condition of the liver can be reversed. They are running additional tests, but right now, it looks like I'm just paying the price for prior years' poor diet and some genetic pre-dispositions.
Tuesday, December 22, 2009
Monday, December 7, 2009
Git 'r Dun
Really good news this week!
My neutrophils jumped from 1.29 last week all the way up to 1.70, and my liver enzymes dropped by more than 10%. The AST level is back to normal. Everything seems to be moving in the right direction, so I think I'll stick with the vitamin and chamomille cocktail I described in my previous post. I'm still doing the coffee/caffeine thing too.
As I write this post, I'm getting my 5th infusion of Rituxan. Prior to treatment, they drew blood for a FACS analysis to count the peripheral hairies, but I'm very confident they won't find any this time.
I'll add plots of the last 3 weeks' blood counts tonight, when I can get to a computer.
Here they are. As you can see most of the counts are back up to the levels they were at just prior to my first Rituxan treatment, when they had already peaked and had started to trend downward. Let's hope the upward momentum keeps going.







My neutrophils jumped from 1.29 last week all the way up to 1.70, and my liver enzymes dropped by more than 10%. The AST level is back to normal. Everything seems to be moving in the right direction, so I think I'll stick with the vitamin and chamomille cocktail I described in my previous post. I'm still doing the coffee/caffeine thing too.
As I write this post, I'm getting my 5th infusion of Rituxan. Prior to treatment, they drew blood for a FACS analysis to count the peripheral hairies, but I'm very confident they won't find any this time.
I'll add plots of the last 3 weeks' blood counts tonight, when I can get to a computer.
Here they are. As you can see most of the counts are back up to the levels they were at just prior to my first Rituxan treatment, when they had already peaked and had started to trend downward. Let's hope the upward momentum keeps going.







Monday, November 30, 2009
Boring is Good
Just finished my 4th cycle of Rituxan (gen. Rituximab), and it was extremely uneventful. Good thing too because I didn't take any Benadryl pre-meds this time. Infusion time was almost exactly three hours. This allowed me to stay alert, do some work on my iPhone, and now I'm about to go back to work at the office. Hopefully I can get 5 hours in before the day is done.
Basic dose information for me is as follows:
Total Dose: 803 mg
Concentration: 2 mg/mL
Dose Rate: initially at 50 mL/hr (100 mg/hr) increasing by 50 mL/hr every 15-30 minutes to 200 mL/hr (400 mg/hr).
The Rituxan total dose is calculated as follows:

My counts are pretty much holding steady for now, in a good range but not yet back up to remission levels. My liver enzymes crept up another 10 to 20%. It seems like nothing I do to make them better seems to work. I've read that people with Crohn's disease secrete high levels of TNFa and that TNFa is also a bone marrow suppressant. Perhaps other digestive issues such as gluten intolerance and Celiac also contribute to high TNFa levels independent of the presence of HCL. If so, then perhaps eliminating chamomile, vitamin A, vitamin E and Omega supplements in hopes of improving my liver function panel actually increased my residual TNFa levels and thus my liver enzymes too (the opposite effect of what I wanted). Then again, perhaps I'm just too impatient and overanalyze without enough data. I've asked for a cytokine panel, or at least a TNFa measurement, but have not heard back yet.
This week, I'm to going to take a vitamin D (1000 iu), E (200 iu) and an Omega-3-6-9 supplement twice daily (total 2000, 400 and 2) and drink chamomile three times a day to suppress TNFa and see if my counts go from holding steady to a pronounced improvement. I'll also take a daily multivitamin in the morning.
Overall it was a boring day, and boring is good.
Basic dose information for me is as follows:
Total Dose: 803 mg
Concentration: 2 mg/mL
Dose Rate: initially at 50 mL/hr (100 mg/hr) increasing by 50 mL/hr every 15-30 minutes to 200 mL/hr (400 mg/hr).
The Rituxan total dose is calculated as follows:

My counts are pretty much holding steady for now, in a good range but not yet back up to remission levels. My liver enzymes crept up another 10 to 20%. It seems like nothing I do to make them better seems to work. I've read that people with Crohn's disease secrete high levels of TNFa and that TNFa is also a bone marrow suppressant. Perhaps other digestive issues such as gluten intolerance and Celiac also contribute to high TNFa levels independent of the presence of HCL. If so, then perhaps eliminating chamomile, vitamin A, vitamin E and Omega supplements in hopes of improving my liver function panel actually increased my residual TNFa levels and thus my liver enzymes too (the opposite effect of what I wanted). Then again, perhaps I'm just too impatient and overanalyze without enough data. I've asked for a cytokine panel, or at least a TNFa measurement, but have not heard back yet.
This week, I'm to going to take a vitamin D (1000 iu), E (200 iu) and an Omega-3-6-9 supplement twice daily (total 2000, 400 and 2) and drink chamomile three times a day to suppress TNFa and see if my counts go from holding steady to a pronounced improvement. I'll also take a daily multivitamin in the morning.
Overall it was a boring day, and boring is good.
Tuesday, November 24, 2009
The Vanishing Point
All I can say is Wow!
After one treatment with Rituxan, FACS results indicate that the percentage of hairy cells in my peripheral bloodstream plummeted from .4% to .05% -- a reduction of 87.5%! I can only hope that it's having the same effect on my marrow infiltration, but I believe it is.
I've now had 3 treatments with Rituxan, so one can expect that the percentage of hairies has now reduced to undectable levels with the standard FACS MRD detection technique. Blood for the next FACS will be taken prior to the Week 5 treatment, so we should know those results in about 3 weeks. It's likely the malignancy will be undetectable!
I also had my Pentamadine (lung antibiotic) treatment yesterday, and it went very well too. The taste was slightly bitter but tolerable. The Pentamadine is a nebulized once monthly treatment in lieu of Bactrim, to prevent the possibility of respiratory infection while being treated.
I'm still trying to figure out why my liver enzymes are elevated. I've been off Bactrim for 10 days, but my last chem20 panel indicated that both the AST and ALT liver enzyme levels went up slightly. I avoid all pain-killers except when they're required for my treatment. Gluten intolerance is also associated with tinnitus and elevated liver enzymes. I think the next step is to go strictly 100% gluten free -- pretty much live off fresh vegetables and meat all cooked at home for two weeks and see if the levels drop significantly.
Anyway, the good news is that the Rituxan is working very well. The FACS count after cycle 1 was .35 cells/uL. Assuming there are 6 liters of peripheral blood, this translates to approximately 2.1 million circulating hairy cells. This means the total HCL count went from 24 million down to 2.1 million in just 1 week.
If the kill process is arithmetic, and the kill capacity per cycle is only dependent on the available volume of Rituxan, then the second cycle may have already killed off the remaining circulating hairy cells; but if the reduction rate continues at 87% of the remaining cells per cycle, I'll still have a few circulating cells left in the near term after cycle 8 (the final cycle). As the cumulative concentration of Rituxan in my system continues its hairy cell search and destroy mission in the months that follow, the remaining cells should be annihilated.
Otherwise, if each round is only half as effective as the previous round, then the total peripheral load would be reduced by 95% in the short term after round 8, which is still really good, but may leave 10 to 20 thousand hairies remaining in the peripheral blood and possibly a hundred thousand or so in the marrow. That's why I need the hyper-sensitive MRD test.
Here's a semilog plot of the FACS cell count per micro-liter since I was diagnosed. If my estimates are correct, then at it's peak, I had 471 million circulating hairies. Now they're getting hard to count.

The treatments are going well. Let's hope all the cycles are as effective as the first one was!
I've got a lot to be thankful for this Thanksgiving!
Three down, five to go...
After one treatment with Rituxan, FACS results indicate that the percentage of hairy cells in my peripheral bloodstream plummeted from .4% to .05% -- a reduction of 87.5%! I can only hope that it's having the same effect on my marrow infiltration, but I believe it is.
I've now had 3 treatments with Rituxan, so one can expect that the percentage of hairies has now reduced to undectable levels with the standard FACS MRD detection technique. Blood for the next FACS will be taken prior to the Week 5 treatment, so we should know those results in about 3 weeks. It's likely the malignancy will be undetectable!
I also had my Pentamadine (lung antibiotic) treatment yesterday, and it went very well too. The taste was slightly bitter but tolerable. The Pentamadine is a nebulized once monthly treatment in lieu of Bactrim, to prevent the possibility of respiratory infection while being treated.
I'm still trying to figure out why my liver enzymes are elevated. I've been off Bactrim for 10 days, but my last chem20 panel indicated that both the AST and ALT liver enzyme levels went up slightly. I avoid all pain-killers except when they're required for my treatment. Gluten intolerance is also associated with tinnitus and elevated liver enzymes. I think the next step is to go strictly 100% gluten free -- pretty much live off fresh vegetables and meat all cooked at home for two weeks and see if the levels drop significantly.
Anyway, the good news is that the Rituxan is working very well. The FACS count after cycle 1 was .35 cells/uL. Assuming there are 6 liters of peripheral blood, this translates to approximately 2.1 million circulating hairy cells. This means the total HCL count went from 24 million down to 2.1 million in just 1 week.
If the kill process is arithmetic, and the kill capacity per cycle is only dependent on the available volume of Rituxan, then the second cycle may have already killed off the remaining circulating hairy cells; but if the reduction rate continues at 87% of the remaining cells per cycle, I'll still have a few circulating cells left in the near term after cycle 8 (the final cycle). As the cumulative concentration of Rituxan in my system continues its hairy cell search and destroy mission in the months that follow, the remaining cells should be annihilated.
Otherwise, if each round is only half as effective as the previous round, then the total peripheral load would be reduced by 95% in the short term after round 8, which is still really good, but may leave 10 to 20 thousand hairies remaining in the peripheral blood and possibly a hundred thousand or so in the marrow. That's why I need the hyper-sensitive MRD test.
Here's a semilog plot of the FACS cell count per micro-liter since I was diagnosed. If my estimates are correct, then at it's peak, I had 471 million circulating hairies. Now they're getting hard to count.

The treatments are going well. Let's hope all the cycles are as effective as the first one was!
I've got a lot to be thankful for this Thanksgiving!
Three down, five to go...
Tuesday, November 17, 2009
Rituxan, Week 2 -- Chimeric Boogaloo
I had my second cycle of Rituxan yesterday, and it went very well. I arrived at the NIH/NCI day hospital at 8 am, was lined up by 8:30, did the blood letting thing soon thereafter and had my Benadryl/Tylenol cocktail around 9:30 or so. We waited for the CBC results before starting my treatment at 10 am. It went very quickly, and since my body is now adjusted to Rituxan, I didn't need any Demerol this time. We started out at a rate of 50, then cranked it up to 200 half an hour later once my vitals checked out okay. I was done in 2 hours 45 minutes. A lot faster than the 7.5 hours it took the week before!
I got the pre-treatment CBC results before leaving and was very surprised by how quickly (and high) my platelets had rebounded. When I left 6 days earlier, my platelet count was at 88, but it jumped up to 122 by Monday morning. As you can see in the plot, that's the highest they've been since late May.

All the other counts had also risen significantly to near pre-Rituxan treatment levels. Two down, six to go...
11/18/2009 Update: Just got my pre-Rituxan flow report for the peripheral bloodstream. It indicates that the concentration of hairies in the peripheral bloodstream doubled to .4% from .2% just 1 week prior. Likewise, the count per microLiter also doubled, so the increase was consistent from both percentage and count perspectives. Given the decrease in normal counts that were also observed in the CBCs since remission was suggested, it's likely that my remission was going to just last a few months, if that. Looks like we hit the Rituxan just in time!
I got the pre-treatment CBC results before leaving and was very surprised by how quickly (and high) my platelets had rebounded. When I left 6 days earlier, my platelet count was at 88, but it jumped up to 122 by Monday morning. As you can see in the plot, that's the highest they've been since late May.

All the other counts had also risen significantly to near pre-Rituxan treatment levels. Two down, six to go...
11/18/2009 Update: Just got my pre-Rituxan flow report for the peripheral bloodstream. It indicates that the concentration of hairies in the peripheral bloodstream doubled to .4% from .2% just 1 week prior. Likewise, the count per microLiter also doubled, so the increase was consistent from both percentage and count perspectives. Given the decrease in normal counts that were also observed in the CBCs since remission was suggested, it's likely that my remission was going to just last a few months, if that. Looks like we hit the Rituxan just in time!
Wednesday, November 11, 2009
Of Mice and Men ...
I started Rituxan biotherapy on Monday in accordance with the NIH protocol. Rituxan is a chimeric monoclonal antibody. The Rituxan chimera is a hybrid of antibodies from both human and murine (mouse) antibodies. The CD20 antigen (a unique protein found on B-cells and abundantly on HCL mutant B-cells) is injected into a mouse, encouraging the production of antibodies. Antibody producing cells are then isolated from the spleen of the animal. These are then combined with immortal cells called myeloma cells. This results in a cell line that will go on producing the antibody indefinitely. Further genetic engineering removes the elements of the mouse cell that would normally produce an immune (allergic) reaction if injected into a human.
One of the forms describing the treatment actually said not to receive it if you have reactions to mouse proteins. How would anybody know that? Although there was that time I had rat-on-a-stick at the 1988 Seoul Olympics...
This is the 6-month post-chemo biotherapy to treat minimal residual disease (MRD). I was fortunate in that my tumor burden is very low, so not a lot of tumor lysis and subsequent reaction was to be expected; hence, less reaction than those with higher burdens in the peripheral blood and marrow. The percentage of hairies in my flow cytometry tests are .2% peripheral and 4% marrow (fairly minscule compared to people who rely on Rituxan as a first line treatment because they don't respond to Cladribine).
I was admitted as an in-patient on Sunday and treatment started on Monday morning. About 7:30 am, a phlebotomist came up to my room and drew blood for the pre-treatment CBC, chem20 and several other tests. Later in the morning, my IV line was placed. Even though I have good veins, they put an order in with the procedures unit to place my IV line using ultrasound. The nurse put the gel on my arm, swiped my arm, picked a vein and placed the line in under a minute. She was able to see the position of the catheter the entire time, so I didn't have to worry about it running into the side of a vein wall or valve. Nice!
My nurse, who was great, reviewed the purpose of the drug and possible side effects, the most common of which are chills, shakes and fever. Pre-meds included Benadryl and Tylenol and the initial Rituxan dosage rate was very low. The going in plan was 12.5 units (can't recall the precise unit label) steady for the first 4 hours, up it to 25 units at hour 4, then increase by 25 units every half hour after that unless reactions were seen. I was great the first hour, then I started to feel mild chills. I tried to fight it, but once I curled up under a blanket, I gave myself away and my nurse decided it was time to give me Demerol after a mild scolding for not telling her sooner. Demerol's a wonder narcotic for knocking out chills (among other things). I was fine after 15 minutes and after that, things went really well.
I took another Benadryl/Tylenol cocktail at hour 4. 10 minutes after each dosage increase, my temperature would go up (max'd at 38.6 C) but it would settle back down to the 37 to 37.5 range before the next increase. Vitals were taken every half hour prior to the dosage increases. Blood pressure was generally in the 118/69 range, pulse was normal and oxygen was anywhere from 95% to 98%. I finished the treatment in 7.5 hours.
I highly recommend that anyone receiving Rituxan biotherapy for HCL discuss Demerol as a option with their doctors. I think I would have suffered a lot without it and think it may help a lot of people who've generally just been treated with high doses of Benadryl. Dr. K is generally available to discuss his results and opinions. I'm sure he'd be happy to talk with your doctor.
I was discharged the next day and feel great, although my counts definitely took a dive. Even though Rituxan targets the CD20 proteins on B-cells, there is generally a broad spectrum affect on all blood counts (at least with the initial cycle) because the immune system goes into a hyper-drive response mode in the presence of the invading mouse protein component of the antibody. I assume the mechanism is some type of elevated phagocytosis but don't know for sure.
One week prior to the Rituxan treatment, I went in for my second bone marrow aspiration in as many weeks. With 4% hairies in the aspirate, I'm really hoping they can get the clone from this one so they can perform the patient specific hyper-sensitive MRD test in the future (1 cell in 1 million vice 1 cell in 10 thousand) when attacking the disease with Rituxan will have the best chance of eradicating it.
In other news, my liver enzyme tests have been consistently high for the past 3 months, so I'm starting to become more concerned. Tests for hepatitis antibodies have all been negative (thank goodness) so one possible remaining optionis that it's a temporary drug reaction caused by the Bactrim. I wrote to Dr. K asking if we could investigate other antibiotics, and he was very receptive and recommended a once-monthly inhaled drug in lieu of the Bactrim. We'll see if that returns the liver enzyme levels to normal.
Residual gluten in additives to the food I eat may be another factor contributing to the elevated liver enzyme levels. Many celiacs have very high liver enzyme levels prior to diagnosis as do people who are gluten intolerant. While I avoid gluten as much as possible, I'm not diligent in avoiding it all the time.
Interestingly enough, the product description for Bactrim also describes the fact that it sometimes has bone marrow suppressive effects, and I've seen anecdotal discussions that some patients have severe adverse reactions when they combine Bactrim and caffeine. Now I'm wondering if Bactrim was suppressing my marrow response after chemotherapy, and my recent experiments with caffeine to lower TNFa actually counter-acted a possible immune suppression effect Bactrim had on my marrow (I know, I know -- crazy mad-scientist and his theories). It'll be interesting to see if switching to the alternate antibiotic causes a dramatic acceleration in my future blood count responses.
Here're some plots of my most recent blood counts. Once again, my neutrophil count dropped when I reduced my coffee and chamomile intake in hopes of lowering my liver enzyme levels in preparation for the Rituxan therapy. Of course one day after Rituxan, everything took a nose dive, especially the lymphocytes. That's good, because the nasty hairies are part of the lymphocyte line of cells.
I've got seven cycles (1 per week) of Rituxan remaining. I'll keep you posted.







One of the forms describing the treatment actually said not to receive it if you have reactions to mouse proteins. How would anybody know that? Although there was that time I had rat-on-a-stick at the 1988 Seoul Olympics...
This is the 6-month post-chemo biotherapy to treat minimal residual disease (MRD). I was fortunate in that my tumor burden is very low, so not a lot of tumor lysis and subsequent reaction was to be expected; hence, less reaction than those with higher burdens in the peripheral blood and marrow. The percentage of hairies in my flow cytometry tests are .2% peripheral and 4% marrow (fairly minscule compared to people who rely on Rituxan as a first line treatment because they don't respond to Cladribine).
I was admitted as an in-patient on Sunday and treatment started on Monday morning. About 7:30 am, a phlebotomist came up to my room and drew blood for the pre-treatment CBC, chem20 and several other tests. Later in the morning, my IV line was placed. Even though I have good veins, they put an order in with the procedures unit to place my IV line using ultrasound. The nurse put the gel on my arm, swiped my arm, picked a vein and placed the line in under a minute. She was able to see the position of the catheter the entire time, so I didn't have to worry about it running into the side of a vein wall or valve. Nice!
My nurse, who was great, reviewed the purpose of the drug and possible side effects, the most common of which are chills, shakes and fever. Pre-meds included Benadryl and Tylenol and the initial Rituxan dosage rate was very low. The going in plan was 12.5 units (can't recall the precise unit label) steady for the first 4 hours, up it to 25 units at hour 4, then increase by 25 units every half hour after that unless reactions were seen. I was great the first hour, then I started to feel mild chills. I tried to fight it, but once I curled up under a blanket, I gave myself away and my nurse decided it was time to give me Demerol after a mild scolding for not telling her sooner. Demerol's a wonder narcotic for knocking out chills (among other things). I was fine after 15 minutes and after that, things went really well.
I took another Benadryl/Tylenol cocktail at hour 4. 10 minutes after each dosage increase, my temperature would go up (max'd at 38.6 C) but it would settle back down to the 37 to 37.5 range before the next increase. Vitals were taken every half hour prior to the dosage increases. Blood pressure was generally in the 118/69 range, pulse was normal and oxygen was anywhere from 95% to 98%. I finished the treatment in 7.5 hours.
I highly recommend that anyone receiving Rituxan biotherapy for HCL discuss Demerol as a option with their doctors. I think I would have suffered a lot without it and think it may help a lot of people who've generally just been treated with high doses of Benadryl. Dr. K is generally available to discuss his results and opinions. I'm sure he'd be happy to talk with your doctor.
I was discharged the next day and feel great, although my counts definitely took a dive. Even though Rituxan targets the CD20 proteins on B-cells, there is generally a broad spectrum affect on all blood counts (at least with the initial cycle) because the immune system goes into a hyper-drive response mode in the presence of the invading mouse protein component of the antibody. I assume the mechanism is some type of elevated phagocytosis but don't know for sure.
One week prior to the Rituxan treatment, I went in for my second bone marrow aspiration in as many weeks. With 4% hairies in the aspirate, I'm really hoping they can get the clone from this one so they can perform the patient specific hyper-sensitive MRD test in the future (1 cell in 1 million vice 1 cell in 10 thousand) when attacking the disease with Rituxan will have the best chance of eradicating it.
In other news, my liver enzyme tests have been consistently high for the past 3 months, so I'm starting to become more concerned. Tests for hepatitis antibodies have all been negative (thank goodness) so one possible remaining optionis that it's a temporary drug reaction caused by the Bactrim. I wrote to Dr. K asking if we could investigate other antibiotics, and he was very receptive and recommended a once-monthly inhaled drug in lieu of the Bactrim. We'll see if that returns the liver enzyme levels to normal.
Residual gluten in additives to the food I eat may be another factor contributing to the elevated liver enzyme levels. Many celiacs have very high liver enzyme levels prior to diagnosis as do people who are gluten intolerant. While I avoid gluten as much as possible, I'm not diligent in avoiding it all the time.
Interestingly enough, the product description for Bactrim also describes the fact that it sometimes has bone marrow suppressive effects, and I've seen anecdotal discussions that some patients have severe adverse reactions when they combine Bactrim and caffeine. Now I'm wondering if Bactrim was suppressing my marrow response after chemotherapy, and my recent experiments with caffeine to lower TNFa actually counter-acted a possible immune suppression effect Bactrim had on my marrow (I know, I know -- crazy mad-scientist and his theories). It'll be interesting to see if switching to the alternate antibiotic causes a dramatic acceleration in my future blood count responses.
Here're some plots of my most recent blood counts. Once again, my neutrophil count dropped when I reduced my coffee and chamomile intake in hopes of lowering my liver enzyme levels in preparation for the Rituxan therapy. Of course one day after Rituxan, everything took a nose dive, especially the lymphocytes. That's good, because the nasty hairies are part of the lymphocyte line of cells.
I've got seven cycles (1 per week) of Rituxan remaining. I'll keep you posted.







Thursday, October 29, 2009
Miracles Happen!!!
Good news! I had a bone marrow biopsy (BMB) and blood tests on Monday and Dr. K says my critical blood counts are all above the minimum thresholds for remission (even if the platelets and neutrophils are below the low-end normal standards). For remission, they use a platelet count of 100K vice 160K. For neutrophils, they use 1.5 vice 1.73. Still, we must get the results of my flow cytometry to determine the level of malignant cells still in the peripheral bloodstream before we can say for sure that it's a complete remission (CR).
The percent infiltration in my marrow is now 30% -- down from a peak of 80% one month after chemo. This is great news, but the level of infiltration is still very high, and I wouldn't be surprised if I relapsed within 20 months. I want to do something about it -- take Rituxan and eradicate it. Unfortunately, I believe the presence of disease in the marrow is used as a control for comparative analysis, not as a qualification for determination of Minimal Residual Disease (MRD) and treatment with Rituxan.
MRD testing is still in its infancy and uses less invasive flow cytometry of peripheral blood in lieu of bone marrow biopsy. Flow cytometry of bone marrow aspirate (BMA) can also be performed, but that is invasive. The goal is to make bone marrow biopsies after the first chemotherapy treatment for Hairy Cell obsolete by developing reliable hyper-sensitive MRD tests along with some other proprietary techniques. Hence, if my flow test does not show MRD, treatment with Rituxan will wait until it does, even though we know there is still significant disease in the marrow. This means the number hairies in the marrow may increase, although they very well may continue to decrease at this point.
The truth is I want my flow to show MRD so I can zap the hairies in the marrow ASAP. If the hairies in the marrow are continuing to die off, then the Rituxan will accelerate the process. If they have already nadired and are on the way back up, then I definitely want to hit them while they're still down.
Now for the bad news. They want more bone marrow aspirate before they treat me with the Rituxan so I've got to go back in for another bone marrow aspiration. That'll be my fourth in the past 7 months. I'm anxious to do it though, because I think there's a good chance it will help a lot with evaluating MRD in the future.
Here's a plot showing a very dramatic increase in the number of neutrophils since last week:
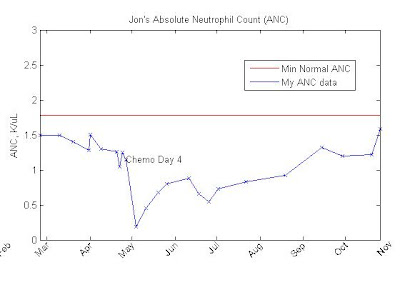
The NCI threshold for remission is 1.5. As you can see, my neutrophils are now at their highest level since I've been collecting CBC data. I'm very relieved, but I'm ready to keep going.
I'll keep you posted.
Update: I just received the peripheral and BMA flow results. The percentage of hairies in the peripheral bloodstream is down to .2%. The percentage in the marrow aspirate is 4%. Thus, MRD has been detected at 6 months post-chemo. I'll go in for another BMA procedure on Monday. The current plan is to see if the BMA hairies will clone before starting Rituxan. This will take a week, so we'll hold off treatment until November 9th.
The percent infiltration in my marrow is now 30% -- down from a peak of 80% one month after chemo. This is great news, but the level of infiltration is still very high, and I wouldn't be surprised if I relapsed within 20 months. I want to do something about it -- take Rituxan and eradicate it. Unfortunately, I believe the presence of disease in the marrow is used as a control for comparative analysis, not as a qualification for determination of Minimal Residual Disease (MRD) and treatment with Rituxan.
MRD testing is still in its infancy and uses less invasive flow cytometry of peripheral blood in lieu of bone marrow biopsy. Flow cytometry of bone marrow aspirate (BMA) can also be performed, but that is invasive. The goal is to make bone marrow biopsies after the first chemotherapy treatment for Hairy Cell obsolete by developing reliable hyper-sensitive MRD tests along with some other proprietary techniques. Hence, if my flow test does not show MRD, treatment with Rituxan will wait until it does, even though we know there is still significant disease in the marrow. This means the number hairies in the marrow may increase, although they very well may continue to decrease at this point.
The truth is I want my flow to show MRD so I can zap the hairies in the marrow ASAP. If the hairies in the marrow are continuing to die off, then the Rituxan will accelerate the process. If they have already nadired and are on the way back up, then I definitely want to hit them while they're still down.
Now for the bad news. They want more bone marrow aspirate before they treat me with the Rituxan so I've got to go back in for another bone marrow aspiration. That'll be my fourth in the past 7 months. I'm anxious to do it though, because I think there's a good chance it will help a lot with evaluating MRD in the future.
Here's a plot showing a very dramatic increase in the number of neutrophils since last week:

The NCI threshold for remission is 1.5. As you can see, my neutrophils are now at their highest level since I've been collecting CBC data. I'm very relieved, but I'm ready to keep going.
I'll keep you posted.
Update: I just received the peripheral and BMA flow results. The percentage of hairies in the peripheral bloodstream is down to .2%. The percentage in the marrow aspirate is 4%. Thus, MRD has been detected at 6 months post-chemo. I'll go in for another BMA procedure on Monday. The current plan is to see if the BMA hairies will clone before starting Rituxan. This will take a week, so we'll hold off treatment until November 9th.
Tuesday, October 20, 2009
Live Hard
We've finally reached the 6 month post-chemo mark, and I'm feeling good. Since last month's post, I've had two CBCs and blood chemistry tests with some very interesting anecdotal evidence regarding caffeine and blood counts.
After my testing in August, I blogged about some research which indicated that caffeine can lower TNFa levels and hypothesized that since some research indicates that HCL apparently thrives on TNFa, maybe drinking coffee and consuming other TNFa lowering foods may improve or sustain my response.
The results from August to September were markedly improved. Upon seeing my liver function test results in September, I stopped taking the neurology drugs for my tinnitus and stopped drinking coffee and omega-3 supplements (fish oil has also been shown to lower TNFa). I was asked to come in two weeks later (September 29th) for a another blood sample so they could try to clone my hairies for PCR one last time before Rituxan treatments start, and they offered to do another CBC and chemistry as well (Nurse R went out of her way to arrange this for me). As shown in the plots below, my red counts, platelets and ANC all decreased in the two weeks between September 14th and September 29th, when I had ceased the coffee, chamomile tea and fish oil.
After getting the September 29th results, I decided to start taking the coffee, tea and fish oil supplements again before my next test, which was today. As you can see, there was improvement in all the counts from September 29th to October 23rd. Obviously this data is only anecdotal but nonetheless interesting in that it correlates with the presence of the anti-TNFa beverages and supplements.







Anyway, I'm now the proud owner of a Keurig single cup coffee brewer. I may even buy one for my office so I can avoid the battery acid that they try to pawn off as coffee. During the daytime, my co-workers know me as mild-mannered Mr. Coffee, not knowing my secret identity -- Java Man -- killer of hairy buggers everywhere (at least the ones in my bone marrow).
Tomorrow, I go in for my 6 month MRI, and my bone marrow biopsy (BMB) is scheduled for Monday, the 26th. Assuming it will take a week to get the pathology report, I expect to start Rituxan either Monday November 2nd, or the following Monday, November 9th. The first round will be administered over an 8 to 10 hour period, and I'll stay at the hospital overnight to make sure I don't have any adverse reactions. Many patients develop fever and vomit during the first round, but most respond with minimal side effects. After the first round, the time to administer subsequent rounds decreases to around 4 hours.
With respect to my daily activities, life is as normal as it's ever been. I haven't had a nose bleed in months, and even though my neutrophils are low, I still engage in pretty much all the activities I would have were I HCL-free. I try to bike 8 miles on Tuesday and Thursday and anywhere from 18 to 20 on Saturday. We've been to several county fairs and recently took Claire to a local Fall festival where we saw pigs and milking cows, rode slides and took a hay ride. I've added some pictures for everyone to enjoy.





Likewise, I'm pleased to announce that baby #2 is now on the way and doing very well -- just in time prior to starting the Rituxan. Once the Rituxan starts, natural conception must be avoided for at least 1 year. In the immortal words of Andy Dufresne: "Get busy living, or get busy dying." I choose to live hard.
After my testing in August, I blogged about some research which indicated that caffeine can lower TNFa levels and hypothesized that since some research indicates that HCL apparently thrives on TNFa, maybe drinking coffee and consuming other TNFa lowering foods may improve or sustain my response.
The results from August to September were markedly improved. Upon seeing my liver function test results in September, I stopped taking the neurology drugs for my tinnitus and stopped drinking coffee and omega-3 supplements (fish oil has also been shown to lower TNFa). I was asked to come in two weeks later (September 29th) for a another blood sample so they could try to clone my hairies for PCR one last time before Rituxan treatments start, and they offered to do another CBC and chemistry as well (Nurse R went out of her way to arrange this for me). As shown in the plots below, my red counts, platelets and ANC all decreased in the two weeks between September 14th and September 29th, when I had ceased the coffee, chamomile tea and fish oil.
After getting the September 29th results, I decided to start taking the coffee, tea and fish oil supplements again before my next test, which was today. As you can see, there was improvement in all the counts from September 29th to October 23rd. Obviously this data is only anecdotal but nonetheless interesting in that it correlates with the presence of the anti-TNFa beverages and supplements.







Anyway, I'm now the proud owner of a Keurig single cup coffee brewer. I may even buy one for my office so I can avoid the battery acid that they try to pawn off as coffee. During the daytime, my co-workers know me as mild-mannered Mr. Coffee, not knowing my secret identity -- Java Man -- killer of hairy buggers everywhere (at least the ones in my bone marrow).
Tomorrow, I go in for my 6 month MRI, and my bone marrow biopsy (BMB) is scheduled for Monday, the 26th. Assuming it will take a week to get the pathology report, I expect to start Rituxan either Monday November 2nd, or the following Monday, November 9th. The first round will be administered over an 8 to 10 hour period, and I'll stay at the hospital overnight to make sure I don't have any adverse reactions. Many patients develop fever and vomit during the first round, but most respond with minimal side effects. After the first round, the time to administer subsequent rounds decreases to around 4 hours.
With respect to my daily activities, life is as normal as it's ever been. I haven't had a nose bleed in months, and even though my neutrophils are low, I still engage in pretty much all the activities I would have were I HCL-free. I try to bike 8 miles on Tuesday and Thursday and anywhere from 18 to 20 on Saturday. We've been to several county fairs and recently took Claire to a local Fall festival where we saw pigs and milking cows, rode slides and took a hay ride. I've added some pictures for everyone to enjoy.





Likewise, I'm pleased to announce that baby #2 is now on the way and doing very well -- just in time prior to starting the Rituxan. Once the Rituxan starts, natural conception must be avoided for at least 1 year. In the immortal words of Andy Dufresne: "Get busy living, or get busy dying." I choose to live hard.
Monday, September 28, 2009
Still Running Up That Hill
My last blood test was two weeks ago and the results are mixed. On the positive side, all my red counts are now above the low end of normal and my neutrophils are higher than they were the day before I started treatment. This is good news which means the Cladribine must have had an effect on the hairy cells in my marrow. Unfortunately, all of my white counts (including the neutrophils) are still well below the low end of normal, which makes me a minor responder -- part of 5% of patients for whom Cladribine treatment does not result in a complete or partial remission.
The latest FACS results indicate that the level of hairies in my peripheral bloodstream is 0.25% (wrt mononuclear cells), so no change since late July. The binding capacity of my hairies for the anti-CD20 monoclonal antibody Rituximab (aka Rituxan) is still over 100k, which means the Rituxan should work well. Unless a miracle happens between now and my CBC and BMB in October, I will still have minimum residual disease (MRD) and undergo treatment with 8 cycles of Rituxan (once a week for 8 weeks), which Dr. K believes "may eradicate the disease."
I'm on cruise control now. Hopefully, the Cladribine has peeled away enough layers of the onion to let the Rituximab finish the job. The veins leading to my marrow that were once clogged with hairies (I compare it to hairy algae clogging the tubing in a fish tank) should be cleared out and ready to let the Rituximab into my marrow.
Here're my latest CBC plots:







I like the acceleration in the red counts. Maybe all that coffee and chamomile drinking helped after all...
I'm going back in tomorrow to have more blood drawn to see if they can clone my hairies for PCR before we start the Rituxan treatments. I'm not sure if this means none of the prior attempts worked or not. Maybe they just want to ensure that they have a "fresh" clone in case the chemo caused mutations or some sort of genetic natural selection in which the surviving hairies are somewhat altered from the general pre-chemo population of clones that were produced. The clones are used in the PCR process to detect 1 hairy cell from 1 million blood cells vice the current state of the art of 1 in 10,000.
If they get the cloning and PCR detection technique to work, it may lead to earlier detection and use of Rituxan as a standard therapy to attack the disease early on when tolerable doses of Rituxan alone can eradicate it. Of course there is always the argument that if the blood counts haven't been affected, there's no need to treate the disease, but if cure can be demonstrated, then this argument may need to be re-examined.
The latest FACS results indicate that the level of hairies in my peripheral bloodstream is 0.25% (wrt mononuclear cells), so no change since late July. The binding capacity of my hairies for the anti-CD20 monoclonal antibody Rituximab (aka Rituxan) is still over 100k, which means the Rituxan should work well. Unless a miracle happens between now and my CBC and BMB in October, I will still have minimum residual disease (MRD) and undergo treatment with 8 cycles of Rituxan (once a week for 8 weeks), which Dr. K believes "may eradicate the disease."
I'm on cruise control now. Hopefully, the Cladribine has peeled away enough layers of the onion to let the Rituximab finish the job. The veins leading to my marrow that were once clogged with hairies (I compare it to hairy algae clogging the tubing in a fish tank) should be cleared out and ready to let the Rituximab into my marrow.
Here're my latest CBC plots:







I like the acceleration in the red counts. Maybe all that coffee and chamomile drinking helped after all...
I'm going back in tomorrow to have more blood drawn to see if they can clone my hairies for PCR before we start the Rituxan treatments. I'm not sure if this means none of the prior attempts worked or not. Maybe they just want to ensure that they have a "fresh" clone in case the chemo caused mutations or some sort of genetic natural selection in which the surviving hairies are somewhat altered from the general pre-chemo population of clones that were produced. The clones are used in the PCR process to detect 1 hairy cell from 1 million blood cells vice the current state of the art of 1 in 10,000.
If they get the cloning and PCR detection technique to work, it may lead to earlier detection and use of Rituxan as a standard therapy to attack the disease early on when tolerable doses of Rituxan alone can eradicate it. Of course there is always the argument that if the blood counts haven't been affected, there's no need to treate the disease, but if cure can be demonstrated, then this argument may need to be re-examined.
Friday, September 4, 2009
RF Radiation, Cancer and HCL Epidemiology
Here's an interesting article from the Amateur Radio Relay League (ARRL) that discusses RF Radiation Safety and studies that address the association between high-level RF Radiation exposure and cancer:
http://www.wave-guide.org/library/arrl.html
Here's an excerpt from a study of the epidemiology of Hairy Cell Leukemia:
HCL risk was concentrated in white males; there were few black and Asian patients for analysis. Overall, the age-adjusted incidence rate of HCL for men (2.9/million population) was 4.8 times greater than that for women (0.6/million population). Using data from all cancer patients diagnosed during the study period, Jewish men had significantly greater risk of HCL than Protestant men.
For men, the OR was significantly elevated for professional and technical workers (OR = 2.1, P = 0.001); within this category of occupations, risk was significantly elevated for engineers (OR = 4.0, P = 0.0008). HCL patients were more than twice as likely to have multiple primary cancer diagnoses as other cancer patients. Since the majority of the other primary cancer diagnoses occurred prior to (>1 year) or concurrent with (1 year) the HCL diagnosis, this greater frequency of multiple primaries in HCL patients may be due to impaired immune function.
http://www.wave-guide.org/library/arrl.html
Here's an excerpt from a study of the epidemiology of Hairy Cell Leukemia:
HCL risk was concentrated in white males; there were few black and Asian patients for analysis. Overall, the age-adjusted incidence rate of HCL for men (2.9/million population) was 4.8 times greater than that for women (0.6/million population). Using data from all cancer patients diagnosed during the study period, Jewish men had significantly greater risk of HCL than Protestant men.
For men, the OR was significantly elevated for professional and technical workers (OR = 2.1, P = 0.001); within this category of occupations, risk was significantly elevated for engineers (OR = 4.0, P = 0.0008). HCL patients were more than twice as likely to have multiple primary cancer diagnoses as other cancer patients. Since the majority of the other primary cancer diagnoses occurred prior to (>1 year) or concurrent with (1 year) the HCL diagnosis, this greater frequency of multiple primaries in HCL patients may be due to impaired immune function.
Friday, August 28, 2009
The One-Two Knockout
Good news for patients who don't respond to Cladribine. I found a study citation that shows that even for minor/non-responders to 2-CDA, complete remission after treatment with Rituximab was achieved.
A study at the University of Pisa, Pisa Italy, studied a cohort of 10 patients who followed a treatment regimen very similar to the one I'm in at NIH. Patients were first treated with a course of 2-CDA (chemo) followed by Rituximab 6 months later. Here's the study citation:
Purine analogues have dramatically improved the outcome of patients affected by hairy cell leukemia (HCL), although complete eradication of disease was achieved in few cases. The purpose of this study was to evaluate the role of Rituximab in eradicating minimal residual disease (MRD) in HCL patients after a pre-treatment with 2-chloro-deoxy-adenosine (2-CdA). Ten patients received four cycles of Rituximab after administration of Cladribrine. Before starting anti-CD20 antibody, two patients were in complete remission, six in partial remission and two showed no significant response to Cladribrine. All cases resulted IgH-positive. Median time from the last 2-CdA infusion was 5.7 months. Eight of 10 patients [four in partial remission (PR), two in complete remission (CR) and two unresponsive after 2-CdA] were evaluable for response. Two months after the end of anti-CD20 therapy, all evaluated patients presented a complete haematological remission. Moreover, Rituximab increased percentage of molecular remission up to 100% 1 yr after the end of treatment. Interestingly, in all cases but one, including those persistently polymerase chain reaction (PCR)-positive, semi-quantitative molecular analyses showed MRD levels lower than those found before Rituximab administration. Toxicity was very mild. The present results not only confirm the therapeutic effect of Rituximab, but also show its relevance in eradicating MRD in HCL.
The really great news here is that of 8 patients, 2 were non-responders at 6 months post-chemo, yet all achieved complete remission after treatment with Rituximab biological therapy, and the toxicity was very mild.
There is still plenty to hope for, and I'm glad I found the NIH study.
A study at the University of Pisa, Pisa Italy, studied a cohort of 10 patients who followed a treatment regimen very similar to the one I'm in at NIH. Patients were first treated with a course of 2-CDA (chemo) followed by Rituximab 6 months later. Here's the study citation:
Purine analogues have dramatically improved the outcome of patients affected by hairy cell leukemia (HCL), although complete eradication of disease was achieved in few cases. The purpose of this study was to evaluate the role of Rituximab in eradicating minimal residual disease (MRD) in HCL patients after a pre-treatment with 2-chloro-deoxy-adenosine (2-CdA). Ten patients received four cycles of Rituximab after administration of Cladribrine. Before starting anti-CD20 antibody, two patients were in complete remission, six in partial remission and two showed no significant response to Cladribrine. All cases resulted IgH-positive. Median time from the last 2-CdA infusion was 5.7 months. Eight of 10 patients [four in partial remission (PR), two in complete remission (CR) and two unresponsive after 2-CdA] were evaluable for response. Two months after the end of anti-CD20 therapy, all evaluated patients presented a complete haematological remission. Moreover, Rituximab increased percentage of molecular remission up to 100% 1 yr after the end of treatment. Interestingly, in all cases but one, including those persistently polymerase chain reaction (PCR)-positive, semi-quantitative molecular analyses showed MRD levels lower than those found before Rituximab administration. Toxicity was very mild. The present results not only confirm the therapeutic effect of Rituximab, but also show its relevance in eradicating MRD in HCL.
The really great news here is that of 8 patients, 2 were non-responders at 6 months post-chemo, yet all achieved complete remission after treatment with Rituximab biological therapy, and the toxicity was very mild.
There is still plenty to hope for, and I'm glad I found the NIH study.
Wednesday, August 26, 2009
Plan B
A few months back I discussed how HCL thrives in the presence of a cytokine (cell signaling molecule) called tumor necrosis factor alpha (TNFa). Given my less than 3-sigma response to Cladribine, I thought it might be worth investigating foods and medicines that suppress the production of TNFa to help me bide my time.
I asked Dr. K (via e-mail) whether they monitor TNFa in the routine blood tests they perform. He said they used to but found the data to be not very meaningful. I assume this means there was too much variance in the data. I then asked him whether given the overall trend in my data, I'm considered a minor responder. He did not respond to that question.
I had read an article in Tallman and Poliak that discussed how TNFa reducing drugs given in parallel with 2-CdA improved response rates in HCL, so I did my own search regarding foods that lower TNFa and struck gold immediately.
As described in "Caffeine suppresses TNFa production via activation of the cyclic AMP/protein kinase A pathway", Horrigan et al, International Immunopharmacology, Vol. 4, No. 10-11 (October, 2004) pp. 1409-1417 -- coffee can suppress TNFa and thus may be helpful in suppressing the rate of cloning of HCL cells. The most caffeinated food (aside from sugar laden jolt and Red Bull) is restaurant prepared espresso. Here is the paper's abstract:
This study investigated the effect of in vitro exposure to caffeine, and its major metabolite paraxanthine, at concentrations relevant to typical caffeine consumption in humans, on lipopolysaccharide (LPS)-stimulated cytokine production in human whole blood. In addition, a role for the cyclic AMP/protein kinase A (PKA) pathway in the immunomodulatory effect of caffeine was investigated. Diluted whole blood (taken following >/=15 h abstinence from caffeine-containing food and beverages) was preincubated with caffeine or paraxanthine (10-100 microM) and stimulated with LPS (1 proportional, variant g/ml) for 24 h. The proinflammatory cytokines tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-12, and the antiinflammatory cytokine IL-10 were measured in cell-free supernatants. Whilst caffeine and paraxanthine had little or no effect on IL-10, IL-1beta, or IL-12 production, TNF-alpha production was suppressed in all individuals studied. The effect was statistically significant at 100 microM and consistent across seven experiments performed. Although not statistically significant, a similar effect was observed with paraxanthine. Caffeine (100 microM) also increased intracellular cyclic AMP concentrations in LPS-stimulated monocytes isolated from whole blood. Moreover, the effect of caffeine on TNF-alpha production was abolished by pretreatment with the protein kinase A inhibitor Rp-8-Br-cAMPS (10(-4) and 10(-5)M). To conclude, this study demonstrates that concentrations of caffeine that are relevant to human consumption consistently suppress production of the proinflammatory cytokine TNF-alpha in human blood and that this effect is mediated by the cyclic AMP/protein kinase A pathway.
I'm going to add one espresso a day to my morning routine in the hope that it will stop the strong cells from signaling further reproduction and infiltration of my marrow. With any luck, I might be able to tip the balance and hold off the stronger cells from reproducing while the chemo continues to take out the weaker ones. Then the Rituximab can come in and wipe out the cells that the chemo couldn't take out.
Foods containing Lutolein (a flavonoid) like celery, green pepper, and chamomile, also suppress TNFa. Other TNFa inhibitors include Nettle Leaf, and ECGC (found in Green Tea). Vitamin A also appears to help suppress TNFa production, which is also linked to the onset of diabetes ("Vitamin A may suppress type 1 diabetes", L. Crowley, Mar. 31, 2008).
I'll be adding all of these to my regular diet.
I wonder if previous studies of TNFa levels proved to be meaningless because diet can affect the levels. Without a controlled diet, studying TNFa levels may prove futile.
TNFa is also associated with demyelinating disorders such as multiple sclerosis and certain forms of tinnitus. Given the fact that Cladribine is effective in treating MS and HCL, I think a logical hypothesis is that Cladribine may somehow block TNFa signalling pathways, possibly by amplifying a protein kinase pathway. Perhaps in minor responders, there is a genetic difference which reduces this effect. A study of individuals who drank a V8-like beverage for 26 days showed they reduced their TNFa production by 34.4%.
Wish me luck!
I asked Dr. K (via e-mail) whether they monitor TNFa in the routine blood tests they perform. He said they used to but found the data to be not very meaningful. I assume this means there was too much variance in the data. I then asked him whether given the overall trend in my data, I'm considered a minor responder. He did not respond to that question.
I had read an article in Tallman and Poliak that discussed how TNFa reducing drugs given in parallel with 2-CdA improved response rates in HCL, so I did my own search regarding foods that lower TNFa and struck gold immediately.
As described in "Caffeine suppresses TNFa production via activation of the cyclic AMP/protein kinase A pathway", Horrigan et al, International Immunopharmacology, Vol. 4, No. 10-11 (October, 2004) pp. 1409-1417 -- coffee can suppress TNFa and thus may be helpful in suppressing the rate of cloning of HCL cells. The most caffeinated food (aside from sugar laden jolt and Red Bull) is restaurant prepared espresso. Here is the paper's abstract:
This study investigated the effect of in vitro exposure to caffeine, and its major metabolite paraxanthine, at concentrations relevant to typical caffeine consumption in humans, on lipopolysaccharide (LPS)-stimulated cytokine production in human whole blood. In addition, a role for the cyclic AMP/protein kinase A (PKA) pathway in the immunomodulatory effect of caffeine was investigated. Diluted whole blood (taken following >/=15 h abstinence from caffeine-containing food and beverages) was preincubated with caffeine or paraxanthine (10-100 microM) and stimulated with LPS (1 proportional, variant g/ml) for 24 h. The proinflammatory cytokines tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-12, and the antiinflammatory cytokine IL-10 were measured in cell-free supernatants. Whilst caffeine and paraxanthine had little or no effect on IL-10, IL-1beta, or IL-12 production, TNF-alpha production was suppressed in all individuals studied. The effect was statistically significant at 100 microM and consistent across seven experiments performed. Although not statistically significant, a similar effect was observed with paraxanthine. Caffeine (100 microM) also increased intracellular cyclic AMP concentrations in LPS-stimulated monocytes isolated from whole blood. Moreover, the effect of caffeine on TNF-alpha production was abolished by pretreatment with the protein kinase A inhibitor Rp-8-Br-cAMPS (10(-4) and 10(-5)M). To conclude, this study demonstrates that concentrations of caffeine that are relevant to human consumption consistently suppress production of the proinflammatory cytokine TNF-alpha in human blood and that this effect is mediated by the cyclic AMP/protein kinase A pathway.
I'm going to add one espresso a day to my morning routine in the hope that it will stop the strong cells from signaling further reproduction and infiltration of my marrow. With any luck, I might be able to tip the balance and hold off the stronger cells from reproducing while the chemo continues to take out the weaker ones. Then the Rituximab can come in and wipe out the cells that the chemo couldn't take out.
Foods containing Lutolein (a flavonoid) like celery, green pepper, and chamomile, also suppress TNFa. Other TNFa inhibitors include Nettle Leaf, and ECGC (found in Green Tea). Vitamin A also appears to help suppress TNFa production, which is also linked to the onset of diabetes ("Vitamin A may suppress type 1 diabetes", L. Crowley, Mar. 31, 2008).
I'll be adding all of these to my regular diet.
I wonder if previous studies of TNFa levels proved to be meaningless because diet can affect the levels. Without a controlled diet, studying TNFa levels may prove futile.
TNFa is also associated with demyelinating disorders such as multiple sclerosis and certain forms of tinnitus. Given the fact that Cladribine is effective in treating MS and HCL, I think a logical hypothesis is that Cladribine may somehow block TNFa signalling pathways, possibly by amplifying a protein kinase pathway. Perhaps in minor responders, there is a genetic difference which reduces this effect. A study of individuals who drank a V8-like beverage for 26 days showed they reduced their TNFa production by 34.4%.
Wish me luck!
Monday, August 24, 2009
Keep on Truckin'
 I underwent chemotherapy (Cladribine -- aka Leustatin) four months ago and although 99% of the malignant cells in my peripheral blood have died off, my bone marrow response has been very slow. Dr. K wants me to remain on a one-month blood work follow up. As you can see in the graph below, my platelet count is still hovering around 100. I think that the count may be deceptively low because the platelets may be aggregating and fooling the FACS into counting what are multiple aggregate platelets as a single platelet. I'm going to ask Dr. K if he can order a peripheral smear slide examination (direct microscope examination by a pathologist) to see if this might be the case.
I underwent chemotherapy (Cladribine -- aka Leustatin) four months ago and although 99% of the malignant cells in my peripheral blood have died off, my bone marrow response has been very slow. Dr. K wants me to remain on a one-month blood work follow up. As you can see in the graph below, my platelet count is still hovering around 100. I think that the count may be deceptively low because the platelets may be aggregating and fooling the FACS into counting what are multiple aggregate platelets as a single platelet. I'm going to ask Dr. K if he can order a peripheral smear slide examination (direct microscope examination by a pathologist) to see if this might be the case.
 The good news is that my WBC, RBC, neutrophil and other counts continue to increase, although slowly. Some other counts, like Basophils, that were previously imperceivable, have now started registering.
The good news is that my WBC, RBC, neutrophil and other counts continue to increase, although slowly. Some other counts, like Basophils, that were previously imperceivable, have now started registering. Full disclosure -- I took 100 mg of grape seed extract (GSE) per day for a week back in June -- after my platelets had gone to 131, but before the next test showed them crashing back down to 100. Dr. K didn't have a problem with it (probably because he doesn't think it'll do anything), but given GSEs apoptotic effects on Jurkat leukemia cells, I thought it might also help destroy HCL cells too. The studies conducted by City of Hope indicated that GSE wouldn't harm healthy cells; however, I'm concerned that the GSE might have somehow knocked down my bone marrow's progenitor cell production. Still, there hasn't been enough data collected on GSE's effects in humans to know for sure.
I found an article addressing the efficacy of injecting Cladribine intravenously -- "Treatment of hairy cell leukemia with cladribine (2-Cda) by subcutaneous bolus injection: a phase II study," by Rohr et al, Annals of Oncology, 2002. I believe it is the basis for Dr. K's decision to use this method of administration in his clinical trial. The median time to failure for this approach is approximately 38 months. That sounds bad, but I think what it really means is that once a complete remission is achieved, it usually takes 38 months before any malignant cells are detected again. It may take much longer before the marrow and blood counts are affected, requiring a second round of chemotherapy.
Using this approach resulted in an overall remission rate of 97% (76% complete, 21% partial). Complete response requires the dissapearance of all evidence of disease, a return to normal peripheral blood counts, and the absence of hairy cells in the blood stream and the bone marrow. Time to failure is defined as the time between treatment start and progression, relapse, second tumor, or death, whichever occurs first. A partial response also requires a return of all blood counts to normal, but the reduction of cells in the marrow is somewhere between 50 and 99 percent.
PRs and CRs usually occur within 10 weeks after chemotherapy, so I'm bummed because it's been 16 weeks and my blood counts are still below normal and malignant cells, however slight, are still being detected in my bloodstream. That makes me part of the 5% considered minor/no response, so I'm glad I'm in the trial. Hopefully, what the Cladribine doesn't kill, the Rituxan I'm getting in October (once a week for 8 weeks) will.

(More at the bottom of this blog post...)




 I've lost a total of 16 pounds over the last two months -- mostly excess fat. I'm down to 188 pounds and holding steady now. My endurance is great, but I have been feeling dizzy lately. I'm anxious for my next bone marrow biopsy in October and to get started on the Rituxan.
I've lost a total of 16 pounds over the last two months -- mostly excess fat. I'm down to 188 pounds and holding steady now. My endurance is great, but I have been feeling dizzy lately. I'm anxious for my next bone marrow biopsy in October and to get started on the Rituxan.I also have a theory on what may have caused my leukemia. Several fellow HCLers have written to me noting that they are also RF engineers or hobbyists, wondering if there may be a common association between our line of work and the disease. A common factor in all of us is that we experienced high-power RF burns over 10 years ago. Likewise, electrical linemen also seem to have a slightly higher incidence of leukemia. Back in 1998, I received a 20 to 40 Watt RF burn at 137.5 MHz when a fellow engineer indicated he had turned off a transmitter but had not. When I disconnected the transmitter's output cable to reconfigure the system for another test, I received a severe RF burn on my hands that took several weeks to heal. In some people, RF burns may cause cellular mutations and induce HCL, but until some meaningful data is collected to prove this, I won't know for sure.
Regardless, bad things happen every day. You just have to accept it and keep on trucking.
KOT!
Tuesday, August 18, 2009
The Cost of Hairy Cell Leukemia
I reviewed all my insurance statements since my first doctor's appointment that led to my diagnosis and treatment for HCL. The total diagnostic cost billed by the doctors was $19250. Add the "virtual" cost of the NIH provided chemo at $5,000, the 1-month BMB at $1500 and the follow-on CBCs at $2000 along with $2500 for "progeny insurance" and the total is around $30260.
The insurance negotiated diagnostic costs came in at $4120 -- a $15,130 savings vice the doctor charges.
Given that I haven't submitted my "progeny insurance" claims yet, my total out-of-pocket (OOP) expense thus far is $2695. A savings of $42565, which may increase when I submit the other claims. My total OOP expense could be as low as $195. Not bad. Say what you want about insurance companies, but I'm grateful for mine. Without their negotiations and coverage, HCL would have left me bankrupt.
What I don't understand is why the initial doctors costs are so high compared to the negotiated costs. The variance between insured negotiated costs and uninsured non-negotiated costs is beyond reason. That the people who can afford it the least are left paying the most when their health turns for the worse is immoral given the large arbitrary cost fluctuations that exist between the insured and non-insured.
Following up on my last blog, my ALT and AST levels after going off Clonazepam were very good -- 24 and 28, respectively, so I've switched from Clonazepam to Gabapentin, which doesn't metabolize. I go in for my 4-month CBC tomorrow. I'll post the results once I get them.
The insurance negotiated diagnostic costs came in at $4120 -- a $15,130 savings vice the doctor charges.
Given that I haven't submitted my "progeny insurance" claims yet, my total out-of-pocket (OOP) expense thus far is $2695. A savings of $42565, which may increase when I submit the other claims. My total OOP expense could be as low as $195. Not bad. Say what you want about insurance companies, but I'm grateful for mine. Without their negotiations and coverage, HCL would have left me bankrupt.
What I don't understand is why the initial doctors costs are so high compared to the negotiated costs. The variance between insured negotiated costs and uninsured non-negotiated costs is beyond reason. That the people who can afford it the least are left paying the most when their health turns for the worse is immoral given the large arbitrary cost fluctuations that exist between the insured and non-insured.
Following up on my last blog, my ALT and AST levels after going off Clonazepam were very good -- 24 and 28, respectively, so I've switched from Clonazepam to Gabapentin, which doesn't metabolize. I go in for my 4-month CBC tomorrow. I'll post the results once I get them.
Friday, July 31, 2009
3 Months Post-Chemo and Counting
It's hard to believe 3 months have passed since I underwent chemotherapy. I had my last blood tests on July 22nd, and the results were mixed. The good news is that about 99% of the malignant cells in my peripheral blood have been knocked down, and blood count-wise things are not getting worse (although I do have some questions about my T-cell ratios).
Each month the rate of reduction in the malignant cells has decreased by half. The first month 98% of the remaining hairy cells were destroyed, the second month 50% of the remaining hairy cells were destroyed, and the third month 25% of the remaining hairy cells were destroyed. I'm gonna go out on a limb and guess that this month 12.5% of the remaining hairy cells (of which there are very few) will be destroyed.
The bad news is that my blood counts aren't really that much better than last month. My white cell count is still about one-third of normal. Still, I've got 3 more months to improve, so I keep reminding myself I'm only halfway there. My liver function tests (AST and ALT levels) came back very high. I'm assuming this is because of the Clonazepam I've been taking to help me sleep, so I stopped taking it for the past week. I'll stay off it until I have my primary care doc do a liver function test next week.
I've lost about 16 pounds since chemotherapy. I simply can't eat enough while I'm at work to keep up with what my body needs. On the bright side, I'm back to working full days on some very interesting NASA science and technology satellites -- NuSTAR (to research the super-massive black hole at the center of our galaxy) and GLORY (to research Earth's aerosols, clouds and irradiance).
I'm really not too concerned about the low neutrophil count anymore. It seems to be slowly increasing, and from all accounts, I'm more susceptible to infections from germs already in my body than external pathogens. Christi, Claire and I went with our friends to the water park last week and had a great time splashing, sliding and playing in the water. I made sure to slather myself in sunblock spray to maintain my lovely pasty-white complexion. It's my sworn duty as leader of the Pasty Boyz.
The following charts show my blood count progress up to the last blood test:






Typically at 3 months, the WBC count is close enough to normal to warrant only testing the peripheral blood 3 months from now, but in my case, I need to go back in 3 more weeks (1 month since the last one).
On a good note, I can workout on my elliptical and exercise bike much more easily than I used to. About two weeks ago, I rode 20 miles on my exercise bike on interval training at about 75% of the bike's full resistance. I'm very anxious the get back to the Grand Canyon and see what I can do once my RBC recovers to a normal level.
Last June, I hiked an hour into the Canyon and it took me about two hours to hike back out. I had to stop and rest about every 200 feet to catch my breath and keep going up. At that point, I was already probably below normal on platelets, and I'm sure my red count was suffering too. Thank God my friends were there to share their water with me and keep me going.
It was exhilarating to make it out on my own two feet -- knowing that my friends were there to pull me through; but I was left wondering "How come it was so much harder for me than everyone else?" Certainly, sitting for 12 hours a day at work didn't help, but I kept wondering if something else was wrong. I wasn't really dehydrated, my legs just got tired really easily. Two days later, 4 people died when a medivac rescuing a hiker from the Canyon was trying to land and collided with another copter taking off from the Flagstaff Hospital. Without the right friends, that could have been me.
Subscribe to:
Posts (Atom)
